A carbon-12 atom consists of six protons, six neutrons and six electrons. The unified atomic mass unit is defined as the mass of the carbon-12 atom. Calculate:
(a) the mass defect in kilograms
(Mass of a proton , mass of a neutron , mass of an electron .)

Important Questions on Nuclear Physics
A carbon-12 atom consists of six protons, six neutrons and six electrons. The unified atomic mass unit is defined as the mass of the carbon-12 atom. Calculate:
(b) the binding energy
(Mass of a proton , mass of a neutron , mass of an electron .)
A carbon-12 atom consists of six protons, six neutrons and six electrons. The unified atomic mass unit is defined as the mass of the carbon-12 atom. Calculate:
(c) the binding energy per nucleon.
(Mass of a proton , mass of a neutron , mass of an electron .)
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium .
The following equation shows the process:
Calculate:
(a) the change in mass in the reaction
(Mass of , Mass of , Mass of , Mass of )
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium .
The following equation shows the process:
Calculate:
(b) the energy released in the reaction
(Mass of , Mass of , Mass of , Mass of )
The fusion reaction that holds most promise for the generation of electricity is the fusion of tritium and deuterium . The following equation shows the process:
Calculate:
(c) the energy released if one mole of deuterium were reacted with one mole of tritium.
(Mass of , mass of , mass of mass of )
The initial activity a sample of 1 mole of radon-220 is .
Calculate:
(a) the decay constant for this isotope.
The initial activity a sample of 1 mole of radon-220 is .
Calculate:
(b) the half-life of the isotope.
The graph of count rate against time for a sample containing indium-116 is shown.
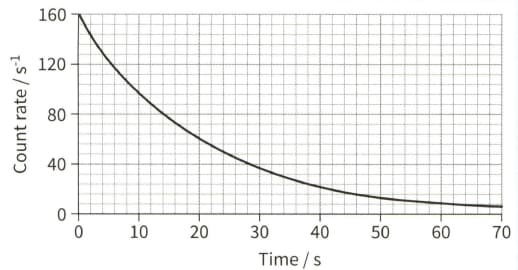
(a) Use the graph to determine the half-life of the isotope.
