MEDIUM
JEE Main
IMPORTANT
Earn 100
A carnot engine working between and has a work output of per cycle. The amount of heat energy supplied to the engine from the source in each cycle is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
A system is neither in thermal equilibrium with nor with . The systems and :
EASY
JEE Main
IMPORTANT
Consider the following thermodynamical variables
(i) Pressure
(ii) Internal Energy
(iii) Volume
(iv) Temperature
Out of these, the intensive variable(s) is (are)
EASY
JEE Main
IMPORTANT
Consider the given diagram. An ideal gas is contained in a chamber (left) of volume and is at an absolute temperature It is allowed to rush freely into the right chamber of volume which is initially vacuum. The whole system is thermally isolated. What will be the final temperature, if the equilibrium has been attained?
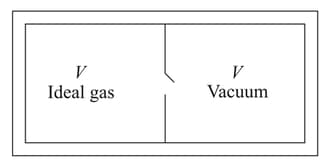
EASY
JEE Main
IMPORTANT
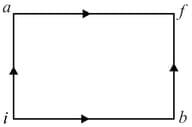
A system is taken from state to state via two different paths.
Path with and
Path
Find the value of (in ) for the second path.
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
