HARD
Earn 100
A catalyst provides a new pathway involving lower amount of activation energy, to make the reaction fast. Which one of the following is/are the result of catalytic action.
(a)Catalyst decreases value
(b)Catalyst increases value.
(c)Catalyst increases value.
(d)Catalyst increases k of reaction
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
EASY
HARD
EASY
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
HARD
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
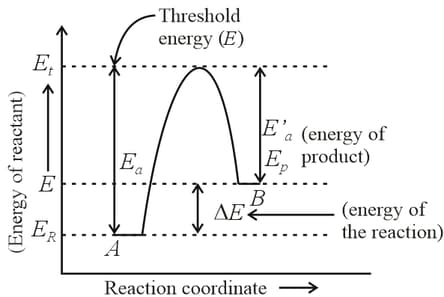
MEDIUM
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
HARD
HARD
Define activation energy.
EASY
MEDIUM
EASY
MEDIUM
in
is equal to __________ . (Integer answer)
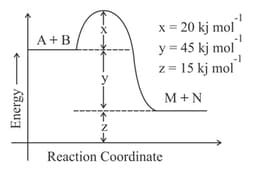
MEDIUM
A first order reaction is completed in minutes at and in minutes at . Calculate the activation energy of the reaction.
().
MEDIUM
Consider the potential energy diagrams of reactions (I) and (II) given below, predict which reaction will go faster and why ?

MEDIUM
MEDIUM
Identify the incorrect statement.
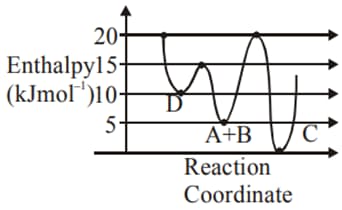
EASY

