EASY
JEE Main/Advance
IMPORTANT
Earn 100
A coal based thermal power plant producing electricity operates between the temperatures and The plant works at of its maximum theoretical efficiency. Complete burning of of coal yields of heat. A house needs units of electricity each day. Coal used for supplying the amount of energy for the house in one year is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
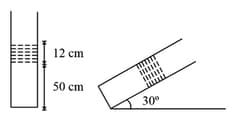
In the given figure, an ideal gas is trapped between a mercury column and the closed end of a uniform vertical tube. The upper end of the tube is open to the atmosphere. Initially, the lengths of the mercury column and the trapped air column are and , respectively. When the tube is tilted slowly in a vertical plane through an angle of with horizontal, then the new length of air column is . Find (assume the temperature to remain constant ).
HARD
JEE Main/Advance
IMPORTANT
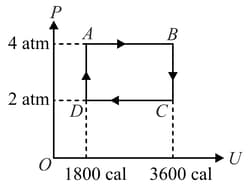
Two moles of an ideal monoatomic gas undergo a cyclic process which is indicated on a diagram where is the internal energy of the gas. The work done by the gas in the cycle is . Find .
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
