A collision between reactant molecules must occur with a certain minimum energy before it is effective in yielding product molecules. This minimum energy is called activation energy ⋅ Larger the value of activation energy, smaller the value of rate constant . Larger is the value of activation energy, greater is the effect of temperature rise on rate constant .
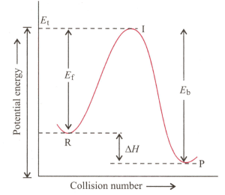
= Activation energy of forward reaction = Activation energy of backward reaction =
Threshold energy
If a reaction is exothermic to the extent of and the forward reaction has an activation energy of the activation energy for reverse reaction in is

Important Questions on Chemical Kinetics
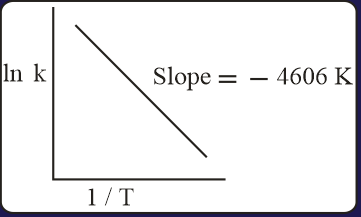
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
The Arrhenius plots of two reactions, I and II are shown graphically-
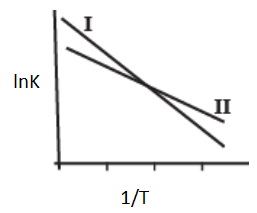
The graph suggests that-
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
[Gas constant, ]
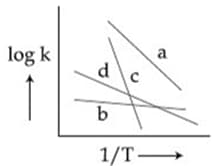
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

Identify the incorrect statement.
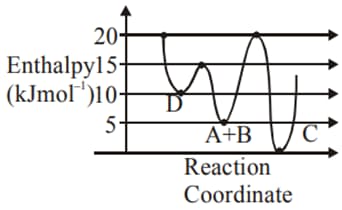
(Assume Activation energy and pre-exponential factor are independent of temperature; )