MEDIUM
Earn 100
A compound contains and only, and has molecular mass Its photochlorination gives a mixture containing only one monochloro and two dichloro hydrocarbons. Deduce the structure of
(a)dimethylpropane
(b) pentane
(c) methyl butane
(d)Cyclopentane
16.67% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
The number of hybridized carbon atoms in
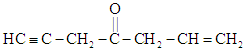
EASY
Give the structural formula of acetic acid.
EASY
Write the Fischer structure of Bromobutane.
MEDIUM
Write the structural formula of propanone.
EASY
In molecule, the hybridization of carbon and respectively, are :
MEDIUM
Write down the structural formula of methanoic acid.
MEDIUM
Draw the structure of propanal.
EASY
The hydrocarbon with seven carbon atoms containing a neopentyl and a vinyl group is:
EASY
Which of the following is an incorrect statement?
MEDIUM
The number of hybridised carbons in an acyclic neutral compound with molecular formula is
EASY
The correct order of catenation is:
EASY
How many pi bonds and sigma bonds are present in following molecule?
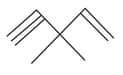
HARD
The chiral compounds among the following are
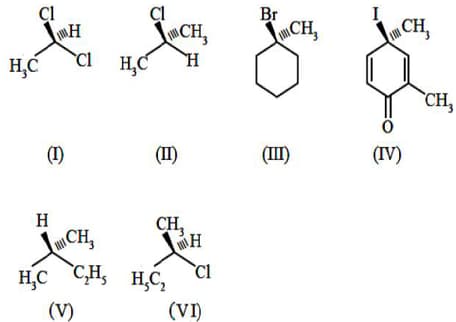
MEDIUM
Draw the structural formula of butanone.
HARD
The functional group present in a molecule having the formula is
EASY
The functional groups that are responsible for the ion-exchange property of cation and anion exchange resins, respectively, are :
EASY
A molecule which has and hydrogen atoms is:
MEDIUM
Give the structural formula of Methylpropanol.
MEDIUM
Which of the following structures contain -hybridised carbon atom(s)?
I.
II.
III. 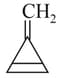
IV. 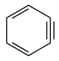
EASY
The number of C - C sigma bonds in the compound


