HARD
Earn 100
A compound having formula weight , has rock salt type structure and the closest AB distance is nm, where is an arbitrary number. The density of lattice is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solid state
EASY
EASY
MEDIUM
HARD
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
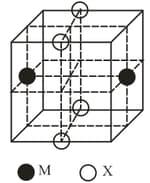
HARD
HARD
MEDIUM
MEDIUM
EASY
EASY
MEDIUM

