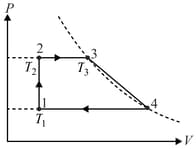
A cyclic process (cycle) consisting of two isobars and , isochor , and a certain process represented by a straight line on the diagram (Fig.) involves moles of an ideal gas. The gas temperatures in states and are , and respectively, and points and lie on the same isotherm. Determine the work done by the gas during the cycle.


Important Questions on Heat and Molecular Physics
Three moles of-an ideal monatomic gas perform a cycle shown in Fig. The gas temperatures in different states are
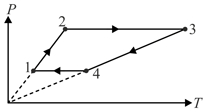
, and .
Determine the work done by the gas luring the cycle.
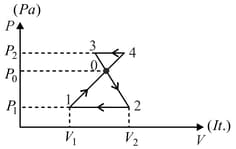
Find the work done by an ideal gas during a closed cycle shown in the figure if and segments and of the cycle are parallel to the -axis?
A gas takes part in two thermal processes in which it is heated from the same initial state to the same final temperature.
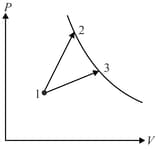
The processes are shown on the diagram by straight lines and (Fig.). Indicate the process in which the amount of heat supplied to the gas is larger.
A vessel of volume is separated into three equal parts by stationary semipermeable thin particles (Fig.). The
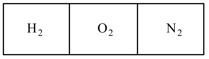
left, middle, and right parts are filled with of hydrogen, of oxygen, and of nitrogen respectively. The left partition lets through only hydrogen, while the right partition lets through hydrogen and nitrogen.
What will be the pressure in each part of the vessel after the equilibrium has been set in if the vessel is kept at a constant temperature ?
A vertical thermally insulated cylinder of volume contains moles of an ideal monatomic gas under a weightless piston.
A load of mass is placed on the piston, as a result of which the piston is displaced by a distance .
Determine the final temperature of the gas established after the piston has been displaced if the area of the piston is and the atmospheric pressure is .
A vertical cylinder of cross-sectional area contains one mole of an ideal monatomic gas under a piston of mass . At a certain instant, a heater which transmits to a gas an amount of heat per unit time is switched on under the piston. Determine the established velocity of the piston under the condition that the gas pressure under the piston is constant and equal to , and the gas under the piston is thermally insulated.
