A cyclic process is depicted on diagram. The and diagrams for this cyclic process are given below. Select the correct choices (more than one options is/are correct)


Important Questions on Laws of Thermodynamics
One mole of a monatomic ideal gas is taken along the cycle as shown in the diagram.
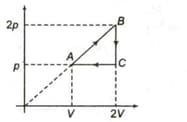
The ratio of specific heat in the process to the specific heat in the process is
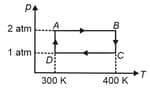
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
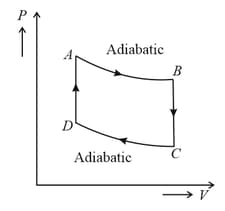
The temperature at is
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
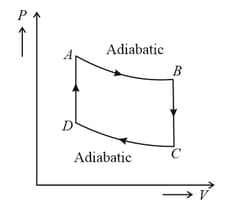
Work done by gas in the process is
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
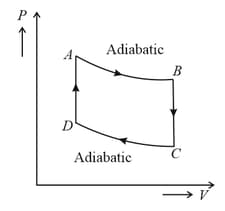
Heat lost by the gas in the process is
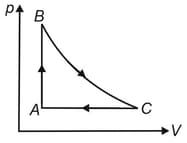
Two moles of helium gas undergoes a cyclic process as shown in the figure. Assuming the gas to be ideal, calculate the following quantities in this process,
The net change in heat energy.
The net work done.
The net change in internal energy.
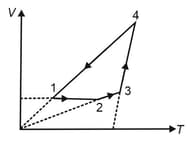
of a sample of helium gas is put through the cycle of operations shown in the figure. is an isothermal process and , , . What are , and ?
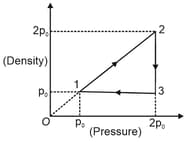
The density versus pressure graph of one mole of an ideal monoatomic gas undergoing a cyclic process is shown in the figure. The molecular mass of gas is .
Find work done in each process.
Find heat rejected by gas in one complete cycle.
Find the efficiency of the cycle.
An ideal gas goes through the cycle . For the complete cycle of heat flows out of the gas. The process is at constant pressure and the process is at constant volume. In the process , . States and have temperatures and .
Sketch the diagram for the cycle. What is the work done by the gas for the process ?