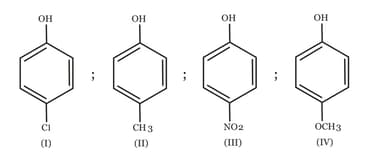
HARD
Earn 100
A delocalized bond is a bond that appears in some forms, but not others.
33.33% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
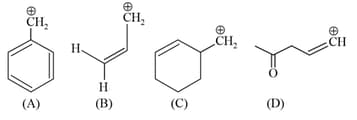
MEDIUM
Which of the following carbocations is expected to be the most stable?
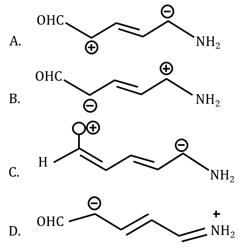
MEDIUM
Among the given species the Resonance stabilised carbocations are:
HARD
Increasing order of stability of the resonance structure is:
EASY
Which of the following statements is not correct for a nucleophile?
EASY
The correct order of stability for the following alkoxides is:
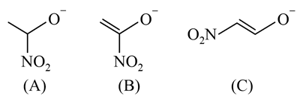
EASY
Identify the functional group that has electron donating inductive effect.
MEDIUM
Which one among the following resonating structures is not correct?
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
EASY
Resonance effect is not observed in
EASY
The most acidic proton and the strongest nucleophilic nitrogen in the following compound
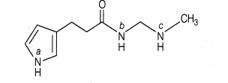
respectively, are
EASY
The order of stability of the following carbocations:
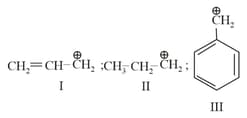
MEDIUM
In which of the following molecules, all atoms are coplanar?
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
EASY
Which of the following is correct with respect to effect of the substituent? (alkyl group)
EASY
Consider the following compounds
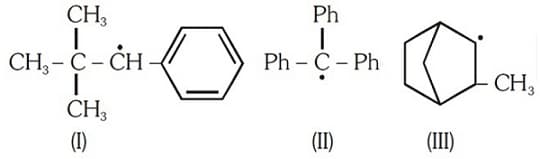
Hyperconjugation occurs in:
MEDIUM
The correct sequence of reagents for the following conversion will be
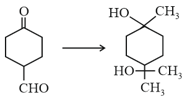
EASY
In pyrrole, the electron density is maximum on
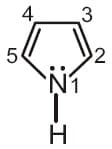
EASY
The chlorine atom of the following compound
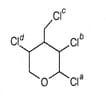
that reacts most readily with to give a precipitate is
MEDIUM
Which of the following molecules is least resonance stabilized?
MEDIUM
Arrange the following compounds in order of decreasing acidity :