MEDIUM
Earn 100
A first order reaction: is performed in a cylinder piston arrangement, at constant temperature and pressure, starting with moles and mole of After minutes from start of reaction, the molar ratio of to becomes The initial volume of system was litre. The correct information(s) related is/are -
(a) of reaction
(b)Volume of system at is
(c)At
(d)At
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
The decomposition reaction is started in a closed cylinder under the isothermal isochoric condition at an initial pressure of . After , the pressure inside the cylinder is found to be . If the rate constant of the reaction is , assuming ideal gas behaviour, the value of is(nearest integer)
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
Which of the following plots is(are) correct for the given reaction?
( is the initial concentration of )
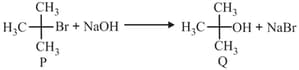
EASY
EASY
MEDIUM
(Take: )
HARD
(Assume that all these gases behave as ideal gases)
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM

