MEDIUM
NEET
IMPORTANT
Earn 100
A first order reaction completes in minutes. The time required for the completion of of the reaction is approx
(a) minutes
(b) minutes
(c) minutes
(d) minutes
100% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
(i) (slow);
(ii) (fast)
The progress of the reaction can be best described by ( is considered as intermediate)
EASY
NEET
IMPORTANT
45. A chemical process occurring in two steps, is plotted as
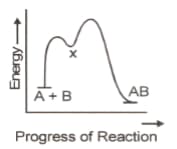
The correct statement is
EASY
NEET
IMPORTANT
For the chemical process energies are plotted in graph.
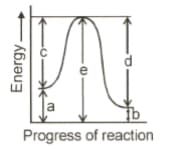
Which of the following is correct ?
