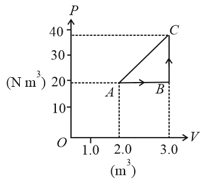
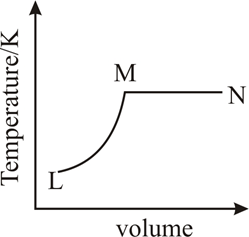
A fixed mass of ideal gas undergoes changes of pressure and volume starting at as shown in figure.
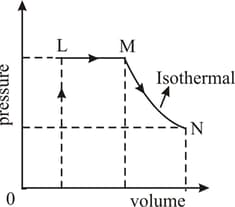
Which of the following is correct :


Important Questions on Thermodynamics
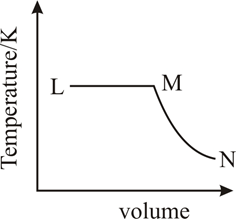
A fixed mass of gas undergoes the cycle of changes represented by as shown in Figure. In some of the changes, work is done on the gas and in others, work is done by the gas. In which pair of the changes work is done on the gas?
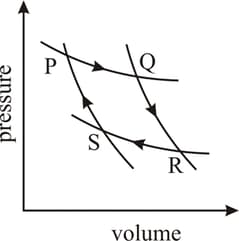
Consider two processes on a system as shown in figure. The volumes in the initial states are the same in the two processes and the volumes in the final states are also the same. Let and be the work done by the system in the processes and , respectively.
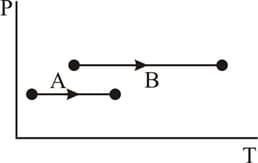
Find work done by the gas in the process shown in figure.
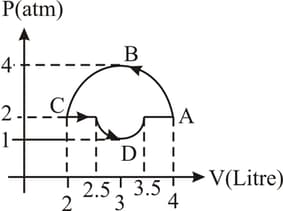
The work done in the following figure is -
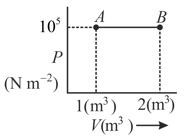
The net amount of the work done in the following indicator diagram is -
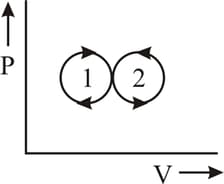
An ideal gas is taken via paths and as shown in fig. The net work done in the whole cycle is-
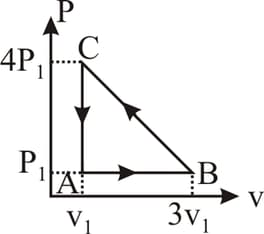
In the indicator diagram shown, the work done along path is -