MEDIUM
JEE Main
IMPORTANT
Earn 100
A flask contains a mixture of compounds and . Both compounds decompose by first-order kinetics. The half-lives for and are and , respectively. If the concentrations of and are equal initially, the time required for the concentration of A to be four times that of (in s) is :
(a)
(b)
(c)
(d)
60% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
JEE Main
IMPORTANT
Higher order (>3) reactions are rare due to:
MEDIUM
JEE Main
IMPORTANT
The number of molecules with energy greater than the threshold energy for a reaction increases five fold by a rise of temperature from to . Its energy of activation in is _________ (Take )
MEDIUM
JEE Main
IMPORTANT
If of a first order reaction was completed in minutes, of the same reaction would be completed in approximately (in minutes) _____
(Take: )
MEDIUM
JEE Main
IMPORTANT
Decomposition of follows a first order reaction. In fifty minutes the concentration of decreases from to in one such decomposition. When the concentration of reaches , the rate of formation of will be:
MEDIUM
JEE Main
IMPORTANT
For the reaction the rate constant (in ) is given by
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
JEE Main
IMPORTANT
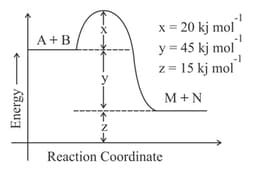
According to the following figure, the magnitude of the enthalpy change of the reaction
in
is equal to __________ . (Integer answer)
MEDIUM
JEE Main
IMPORTANT
For a first order reaction, the ratio of the time for completion of a reaction to the time for completion is ___________ . (Integer answer)
EASY
JEE Main
IMPORTANT
For the reaction , which statement is correct?
