A free neutron decays according to the equation . If the neutron-hydrogen atomic mass difference is , what is the maximum kinetic energy possible for the electron produced in a neutron decay?

Important Questions on Nuclear Physics
The radionuclide decays according to
,
The maximum energy of the emitted positron is .
(a) Show that the disintegration energy for this process is given by,
where, and are the atomic masses of and , respectively, and is the mass of a positron.
(b) Given the mass values, , and , calculate and compare it with the maximum energy of the emitted positron given above. (Hint: Let and be the nuclear masses and then add in enough electrons to use the atomic masses)
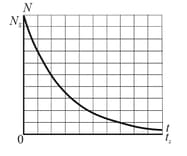
Figure shows the decay of parents in a radioactive sample. The axes are scaled by and . What is the activity of the sample at ?
The isotope decays to with a half-life of . Although the decay occurs in many individual steps, the first step has by far the longest half-life. Therefore, one can often consider the decay to go directly to Lead. That is,
various decay products.
A rock is found to contain of and of . Assume that the rock contained no Lead at formation, so all the Lead present later arose from the decay of Uranium. How many atoms of (a) and (b) does the rock contain later? (c) How many atoms of did the rock contain at formation ? (d) What is the age of the rock?
