EASY
JEE Main
IMPORTANT
Earn 100
A free neutron decays into a proton but a free proton does not decay into neutron. This is because
(a)neutron is an uncharged particle
(b)proton is a charged particle
(c)neutron is a composite particle made of a proton and an electron
(d)neutron has larger rest mass than proton
50% studentsanswered this correctly

Important Questions on Atoms and Nuclei
EASY
JEE Main
IMPORTANT
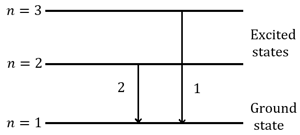
For hydrogen atom, and are the wavelengths corresponding to the transitions and respectively as shown in figure. The ratio of and is . The value of is ______.
EASY
JEE Main
IMPORTANT
The radius of electron's second stationary orbit in Bohr's atom is . The radius of orbit will be
EASY
JEE Main
IMPORTANT
If the binding energy of ground state electron in a hydrogen atom is , then, the energy required to remove the electron from the second excited state of will be: . The value of is _____.
EASY
JEE Main
IMPORTANT
The mass of proton, neutron and helium nucleus are respectively . The binding energy of helium nucleus is:
MEDIUM
JEE Main
IMPORTANT
A light of energy is incident on a hydrogen atom in its ground state. The atom absorbs the radiation and reaches to one of its excited states. The angular momentum of the atom in the excited state is . The value of is ______ (use )
EASY
JEE Main
IMPORTANT
An electron of a hydrogen like atom, having , jumps from energy state to energy state, The energy released in this process, will be: (Given )
Where Rydberg
constant Speed of light in vacuum
Planck's constant
MEDIUM
JEE Main
IMPORTANT
Nucleus having and equal number of protons and neutrons has binding energy per nucleon. Another nucleus of has total nucleons and binding energy per nucleons. The difference of binding energy of and will be ______ .
