A frictionless piston-cylinder based enclosure contains some amount of gas at pressure . Then heat is transferred to the gas at constant pressure in a quasi-static process. The piston moves at slowly through a hight of . In the piston has a cross-section area of , the work done by the gas in this process is

Important Questions on Thermodynamics
A solid of constant heat capacity is being heated by keeping it in contact with reservoirs in two ways :
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoir supplies the same amount of heat. In both the cases body is brought from initial temperature to final . Entropy change of the body in the two cases respectively is :
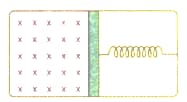
An ideal monoatomic gas is confined in a horizontal cylinder by a spring loading piston (as shown in Fig ). Initially the gas is at temperature pressure and volume and the spring is in relaxed state. The gas is then Fig. heated very slowly to temperature , pressure and volume . During this process the piston moves out by a distance . Ignoring the friction between the piston and the cylinder, the correct statements is (are)
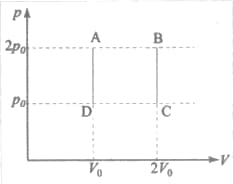
The above diagram Fig. represents the thermodynamic cycle of an engine, operating with an ideal monatomic gas. The amount of heat extracted from the source in a single cycle is :