HARD
Earn 100
A gas expands adiabatically at constant pressure such that :
The value of i.e., of the gas will be :
(a)1.30
(b)1.50
(c)1.70
(d)2
50% studentsanswered this correctly
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
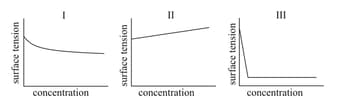
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(Latent heat of ice is and )
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
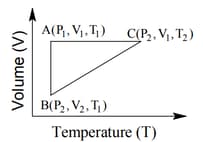
The correct option(s) is (are)
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
The combination of plots which does not represent isothermal expansion of an ideal gas is
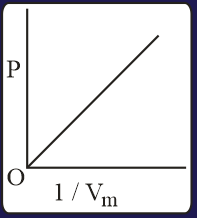
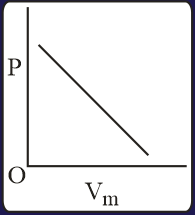
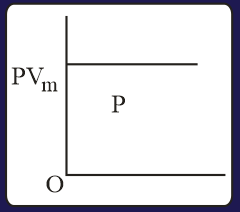
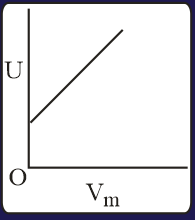
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
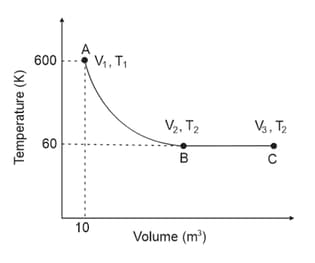
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
Reversible process
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
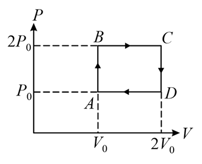
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
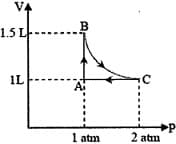
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
[Heat of fusion of ice ; Specific heat of water ]
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(: pressure, : volume, : temperature, : enthalpy, : entropy)
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other

