MEDIUM
Earn 100
A gas expands from a volume of 1 m3 to a volume of 2 m3 against an external pressure of 105 N m-2 . The work done by the gas will be
(a)105 kJ
(b)102 kJ
(c)102 J
(d)103 J
50% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
HARD
In a thermodynamics process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by where is temperature of the system and is the infinitesimal change in a thermodynamic quantity of the system. For a mole of monatomic ideal gas Here, is gas constant, is volume of gas, and are constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| Column - I | Column - II | ||
| (I) | Work done by the system in process | (P) | |
| (II) | Change in internal energy in process | (Q) | |
| (III) | Heat absorbed by the system in process | (R) | |
| (IV) | Heat absorbed by the system in process | (S) | |
| (T) | |||
| (U) |
If the process on one mole of monatomic ideal gas is as shown in the -diagram with the correct match is
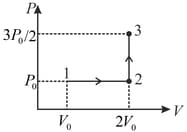
MEDIUM
HARD
In a thermodynamics process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by where is temperature of the system and is the infinitesimal change in a thermodynamic quantity of the system. For a mole of monatomic ideal gas Here, is gas constant, is volume of gas, and are constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| List I | List II | ||
|---|---|---|---|
| (I) | Work done by the system in process | (P) | |
| (II) | Change in internal energy in process | (Q) | |
| (III) | Heat absorbed by the system in process | (R) | |
| (IV) | Heat absorbed by the system in process | (S) | |
| (T) | |||
| (U) |
If the process carried out on one mole of monatomic ideal gas is as shown in figure in the - -diagram with the correct match is,
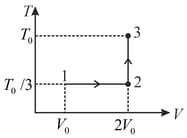
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :
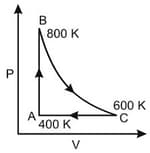
MEDIUM
An ideal gas is taken reversibly around the cycle as shown on the T (temperature) - S (entropy) diagram
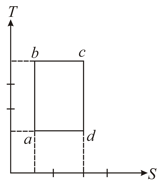
The most appropriate representation of above cycle on a U (internal energy)-V (volume) diagram is
EASY
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
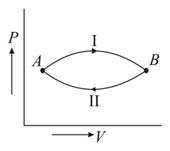
EASY
HARD
MEDIUM
A thermodynamic system undergoes a cyclic process as shown in the diagram. The work done by the system per cycle is
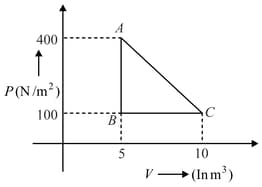
HARD
[ universal gas constant ]
MEDIUM
EASY
MEDIUM
MEDIUM
EASY

