EASY
Physics
IMPORTANT
Earn 100
A gas in container is in thermal equilibrium with another gas in container . Both contain equal masses of the two gases. Which of the following can be true?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
Physics
IMPORTANT
Pick the correct statement(s).
HARD
Physics
IMPORTANT
Two identical containers, each of volume , are joined by a small pipe. The containers contain identical gases at temperature and pressure . One container is heated to temperature , while maintaining the other at the same temperature. The common pressure of the gas is and is the number of moles of gas in a container at temperature . Then,
HARD
Physics
IMPORTANT
A closed vessel contains a mixture of two diatomic gases and . Molar mass of is times that of and mass of gas contained in the vessel is times that of . Choose the correct statement().
HARD
Physics
IMPORTANT
An ideal gas undergoes a thermodynamic cycle as shown in figure.
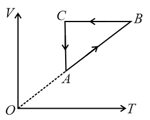
Which of the following graphs may represent the same cycle?
MEDIUM
Physics
IMPORTANT
Which of the following statements are correct?
MEDIUM
Physics
IMPORTANT
An ideal gas is taken from state (pressure , volume ) to state (pressure , volume ) along a straight-line path in the diagram. Select the correct statements from the following.
EASY
Physics
IMPORTANT
An ideal gas undergoes an expansion from a state with temperature and volume through three different polytropic processes and as shown in the diagram. If and be the magnitude of change in internal energy along the three paths respectively, then
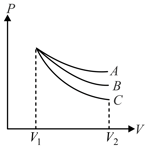
MEDIUM
Physics
IMPORTANT
In the arrangement shown in the given figure, the gas is thermally insulated. An ideal gas is filled in the cylinder having pressure (atmospheric pressure ). The spring of force constant is initially unstretched. The piston of mass and area is frictionless. In equilibrium, the piston rises up by distance , then