EASY
Earn 100
A gas is compressed adiabatically, which one of the following statement is NOT true?
(a)There is no heat supplied to the system
(b)There is no change in the internal energy
(c)
The temperature of the gas increases
(d)The change in the internal energy is equal to the work done on the gas
79.69% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
HARD
(Given is gas constant)
MEDIUM
HARD
MEDIUM
EASY
EASY
EASY
MEDIUM
Two moles of an ideal monoatomic gas occupies a volume at . The gas expands adiabatically to a volume . Calculate the final temperature of the gas and change in its internal energy.
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 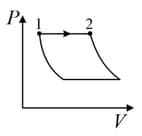 |
| (II) | (ii) Isochoric | (Q) 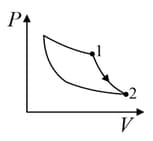 |
| (III) | (iii) Isobaric | (R) 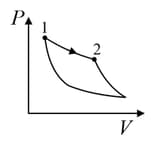 |
| (IV) | (iv) Adiabatic | (S) 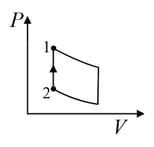 |
EASY
MEDIUM
EASY
HARD
HARD
EASY
HARD
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 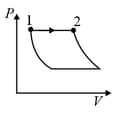 |
| (II) | (ii) Isochoric | (Q) 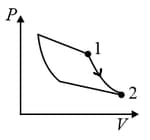 |
| (III) | (iii) Isobaric | (R) 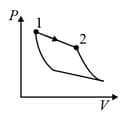 |
| (IV) | (iv) Adiabatic | (S) 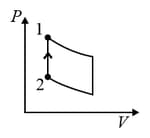 |
HARD
HARD

