MEDIUM
JEE Main
IMPORTANT
Earn 100
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then
(a)compressing the gas isothermally or adiabatically will require the same amount of work.
(b)which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
(c)compressing the gas isothermally will require more work to be done.
(d)compressing the gas through adiabatic process will require more work to be done.
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main
IMPORTANT
A monatomic gas at a pressure , having a volume expands isothermally to a volume and then adiabatically to a volume . The final pressure of the gas is (take )
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
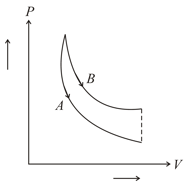
In figure, and are two adiabatic curves for two different gases. Then, and correspond to,
HARD
JEE Main
IMPORTANT
