EASY
NEET
IMPORTANT
Earn 100
A gas is contained in a metallic cylinder fitted with a piston. The piston is suddenly moved in to compress the gas and is maintained at this position. As time passes, the pressure of the gas in the cylinder
(a)Increases
(b)Decreases
(c)Remains constant
(d)Increases or decreases depending on the nature of the gas
18.75% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
Two samples of and are initially kept in the same state. The sample is expanded through an adiabatic process and the sample through an isothermal process up to the same final volume. The final pressures in and are and respectively. Then,
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Four curves and are drawn in the figure for a given amount of gas. The curves which represent adiabatic and isothermal changes are
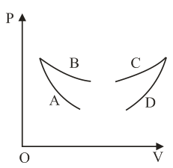
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
When an ideal gas undergoes an adiabatic change causing a temperature change
(i) there is no heat gained or lost by the gas.
(ii) the work done by the gas is equal to a change in internal energy.
(iii) the change in internal energy per mole of the gas is where is the molar heat capacity at constant volume.
