MEDIUM
NEET
IMPORTANT
Earn 100
A gas is expanded from volume to under three different processes. Process is isobaric process, process is isothermal and process is adiabatic. Let and be the change in internal energy of the gas is these three processes. Then:
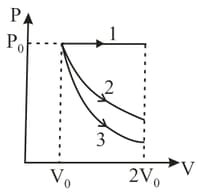

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
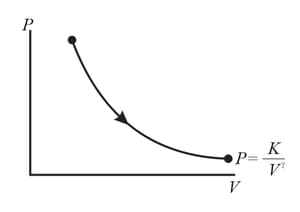
The molar heat capacity for the process shown in figure is (here is a constant)
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
