EASY
Physics
IMPORTANT
Earn 100
A gas is expanded from volume to under three different processes. Process is isobaric process, process is isothermal and process is adiabatic. Let and be the change in the internal energy of the gas is these three processes respectively. Then

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Physics
IMPORTANT
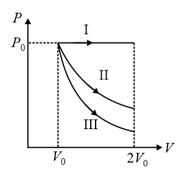
Four curves and are drawn in the figure for a given amount of gas. The curves which represent adiabatic and isothermal process are,
MEDIUM
Physics
IMPORTANT
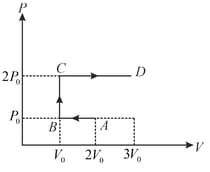
The ideal gas of mass in a state goes to another state via three different processes as shown in the figure. If and denote the heat absorbed by the gas along the three paths, then
EASY
Physics
IMPORTANT
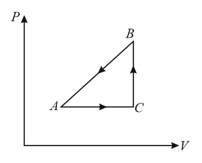
The diagram of a system undergoing thermodynamic transformation is as shown in figure. The work done on the system in going from is and cal heat is given to the system. The change in internal energy between and is
EASY
Physics
IMPORTANT
If universal gas constant, the amount of heat needed to raise the temperature of of an ideal monatomic gas from to , when no work is done is
EASY
Physics
IMPORTANT
A thermally insulated container is divided into two parts by a screen. In one part, the pressure and temperature are and for an ideal gas filled. In the second part, it is vacuum. If now a small hole is created in the screen, then the temperature of the gas will _____.
MEDIUM
Physics
IMPORTANT
Two samples and of a gas initially at the same pressure and temperature are compressed from volume to ( isothermally and adiabatically). The final pressure of is
MEDIUM
Physics
IMPORTANT
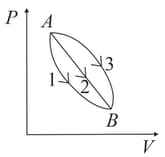
Three processes compose a thermodynamic cycle shown in the accompanying diagram of an ideal gas.
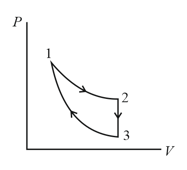
Process takes place at constant temperature, during this process of heat enters the system.
Process takes place at constant volume. During this process of heat leaves the system.
Process is adiabatic.
What is the change in internal energy of the system during process ?
MEDIUM
Physics
IMPORTANT
diagram of an ideal gas is as shown in the figure. Work done by the gas in a process is