MEDIUM
JEE Main
IMPORTANT
Earn 100
A gas mixture consists of moles of oxygen and moles of argon at temperature . Assuming the gases to be ideal and the oxygen bond to be rigid, the total internal energy (in units of ) of the mixutre is :
(a)
(b)
(c)
(d)
72.73% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main
IMPORTANT
An ideal gas in a closed container is slowly heated. As its temperature increases, which of the following statements are true ?
(A) the mean free path of the molecules decreases
(B) the mean collision time between the molecules decreases.
(C) the mean free path remains unchanged.
(D) the mean collision time remains unchanged.
EASY
JEE Main
IMPORTANT
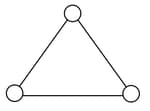
Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:
EASY
JEE Main
IMPORTANT
To raise the temperature of a certain mass of gas by at a constant pressure, calories of heat is required. When the same mass of gas is cooled by at constant volume, calories of heat is released. How many degrees of freedom does each molecule of this gas have (assume gas to be ideal)?
EASY
JEE Main
IMPORTANT
Match the ratio for ideal gases with different type of molecules:
| Molecule Type | |
| (A) Monoatomic | (I) |
| (B) Diatomic rigid molecules | (II) |
| (C) Diatomic non-rigid molecules | (III) |
| (D) Triatomic rigid molecules | (IV) |
HARD
JEE Main
IMPORTANT
Particle of mass moving along the -axis with velocity collides elastically with another particle at rest having mass . If both the particles move along the -axis after the collision, the change in the wavelength of the particle , in terms of its de-Broglie wavelength before the collision is:
EASY
JEE Main
IMPORTANT
A closed vessel contains mole of a monoatomic ideal gas at . If mole of the same gas at is added to it, the final equilibrium temperature (in ) of the gas in the vessel will be close to ______
MEDIUM
JEE Main
IMPORTANT
The change in the magnitude of the volume of an ideal gas when a small additional pressure is applied at a constant temperature, is the same as the change when the temperature is reduced by a small quantity at constant pressure. The initial temperature and pressure of the gas were and respectively. If then value of in is __________
EASY
JEE Main
IMPORTANT
Number of molecules in a volume of of a perfect monoatomic gas at some temperature and at a pressure of of mercury is close to? (Given, mean kinetic energy of a molecule (at T) is density of mercury )
