MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
A gas obeying the equation of state undergoes a hypothetical reversible process described by the equation, , where and are dimensioned constants. Then, for this process, the thermal compressibility at high temperature
(a)approaches a constant value.
(b)is proportional to .
(c)is proportional to .
(d)is proportional to .
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases and Thermodynamics
HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
The ratio of r.m.s. speed to the r.ms. angular speed of a diatomic gas at a certain temperature is: (assume mass of one molecule, molecular mass, moment of inertia of the molecules)
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
A gas mixture consists of moles of oxygen and moles of argon at temperature . Neglecting all vibrational modes, the total internal energy of the system is:
HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
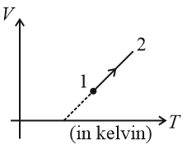
- diagram for a process of a given mass of ideal gas is as shown in the figure. During the process, the pressure of the gas
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
The molecules of an ideal gas have degrees of freedom. The temperature of the gas is . The average translational kinetic energy of its molecules is:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
Four particles have velocities . The root-mean-square velocity of the particles is: (in )
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
In a process, the density of a gas remains constant. If the temperature is doubled, then the change in the pressure will be:
HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
Which of the following will have maximum total kinetic energy at temperature .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Assume a sample of an ideal gas in a vessel. Where velocity of molecules are between and velocity of molecules and number of molecules are related as . The most probable velocity in sample is. where is measured in .
