MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A gaseous mixture enclosed in a vessel contains mole of a gas (with ) and another gas (with ) at a temperature . The gases and do not react with each other and are assumed to be ideal. The number of gram moles of if for the gaseous mixture is is:
(a)
(b)
(c)
(d)
25% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
HARD
JEE Main/Advance
IMPORTANT
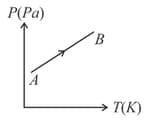
Figure shows diagram for a given mass of an ideal gas for the process During this process, density of the gas is
EASY
JEE Main/Advance
IMPORTANT
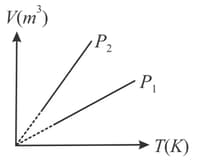
Figure shows curves for a given mass of an ideal gas at pressures and Then
HARD
JEE Main/Advance
IMPORTANT
The diagram of a gas at constant temperature are drawn. The curve is for a constant mass and temperature and curve is for a constant mass and temperature Select the correct alternative.

EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
