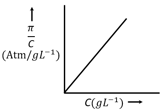MEDIUM
JEE Main
IMPORTANT
Earn 100
A gaseous mixture of two substances and , under a total pressure of is in equilibrium with an ideal liquid solution. The mole fraction of substance is in the vapour phase and in the liquid phase. The vapour pressure of pure liquid is____atm.(Nearest integer)
50% studentsanswered this correctly

Important Questions on Solutions
HARD
JEE Main
IMPORTANT
If gas is bubbled through water at , the number of millimoles of gas that dissolve in litre of water is____(Nearest integer) (Given : Henry's Law constant for at is bar and partial pressure of bar)
(Assume solubility of in water is too small, nearly negligible)
HARD
JEE Main
IMPORTANT
of solute A was dissolved in of ethanol and freezing point of the solution was found to be . The molar mass of solute is . [Given: Freezing point of ethanol is . Density of ethanol is . Freezing point depression constant of ethanol is ]
MEDIUM
JEE Main
IMPORTANT
In the depression of freezing point experiment
A. Vapour pressure of the solution is less than that of pure solvent
B. Vapour pressure of the solution is more than that of pure solvent
C. Only solute molecules solidify at the freezing point
D. Only solvent molecules solidify at the freezing point
MEDIUM
JEE Main
IMPORTANT
The Total pressure observed by mixing two liquid is when their mole fractions are respectively. The Total pressure becomes if the mole fractions are changed to respectively for . The vapour pressure of pure is ______ . (Nearest integer) Consider the liquids and solutions behave ideally
HARD
JEE Main
IMPORTANT
The osmotic pressure of solutions of PVC in cyclohexanone at are plotted on the graph. The molar mass of PVC is (Nearest integer)
(Given : )
MEDIUM
JEE Main
IMPORTANT
The number of pairs of the solution having the same value of the osmotic pressure from the following is
(Assume ionization)
A. and
B. and
C. (aq) and
D. and
E. and
HARD
JEE Main
IMPORTANT
Solid Lead nitrate is dissolved in litre of water. The solution was found to boil at . When of is added to the resulting solution, it was observed that the solution froze at . The solutbility product of formed is _____ at . (Nearest integer)
Given : and . Assume molality to be equal to molarity in all cases.
EASY
JEE Main
IMPORTANT
Match List-I and List-II
| List-I | List-II | ||
| A. | Osmosis | I. | Solvent molecules pass through semi permeable membrane towards solvent side. |
| B. | Reverse osmosis | II. | Movement of charged colloidal particles under the influence of applied electric potential towards oppositely charged electrodes |
| C. | Electro osmosis | III. | Solvent molecules pass through semi permeable membrane towards solution side |
| D. | Electrophoresis | IV. | Dispersion medium moves in an electric field. |
Choose the correct answer from the options given below: