EASY
Earn 100
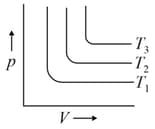
A graph based on Gay-Lussac's law, shows the relationship between pressure and absolute temperature of a gas at constant _____.
(a)Pressure
(b)Volume
(c)Mass of the gas
(d)Temperature
50% studentsanswered this correctly
Important Questions on Atoms and Molecules
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
For the given isotherms, which of the following is correct for
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
[Gas constant, ]
EASY
MEDIUM
MEDIUM
Choose the correct option for graphical representation of Boyle's law, which shows a graph of pressure vs. volume of a gas at different temperatures:
MEDIUM
EASY
EASY
EASY
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
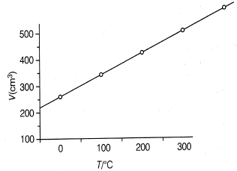
The volume of the gas at is larger than that at by a factor of

