HARD
JEE Main
IMPORTANT
Earn 100
A graph between vs , with being the initial concentration of in the reaction Product, is shown in the figure.
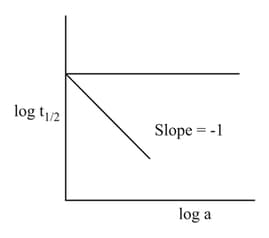
Then the rate law is

(a)
(b)
(c)
(d)
54.17% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
Assuming gas-phase decomposition of dimethyl ether follows order kinetics:
The reaction is carried out in a constant volume container at and has a half-life . Initially, only dimethyl ether is present at a pressure of . The total pressure of the system after is: (given )
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
| [A] | [B] | [C] | Rate |
MEDIUM
JEE Main
IMPORTANT
