MEDIUM
NEET
IMPORTANT
Earn 100
A graph between and concentration for order reaction is a straight line. Reaction of this nature is completed in minutes when concentration is . This is decomposed in minutes at and are respectively
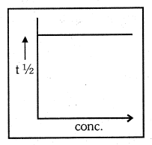

(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
In the first order reaction of the reactant disappeared in . Calculate the rate constant of the reaction :
MEDIUM
NEET
IMPORTANT
In the case of first order reaction, the ratio of time required for completion to completion is:-
EASY
NEET
IMPORTANT
From different sets of data of at different initial concentration say for a given reaction, the product is found to be constant. The order of the reaction is:
MEDIUM
NEET
IMPORTANT
The reaction
is first order with respect to Which of the following graph would yield a straight line :-
MEDIUM
NEET
IMPORTANT
Which of the following statement is not correct for the reaction whose rate is (rate constant)
EASY
NEET
IMPORTANT
Which of the following curves represent a order reaction:
MEDIUM
NEET
IMPORTANT
The following data were obtained at a certain temperature for the decomposition of ammonia
The order of the reaction is :-
MEDIUM
NEET
IMPORTANT
A reaction is found to have the rate constant by what factor the rate is increased if initial concentration of is tripled
