A half cell is prepared by dipping Ag electrode in a solution containing KCl and some AgCl. Half cell is used as cathode during the cell reaction. Quantity of AgCl will –
Important Questions on Electrochemistry
Some materials are given below:
Write the chemical equation fo the reaction taking place at the cathode.
Some materials are given below:
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
,
At , the of the galvanic cell mentioned below is
The reaction occurs in which of the given galvanic cell?
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
Some materials are given below:
Which is the anode of this cell?
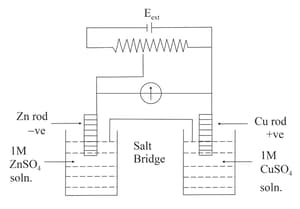
Identify the incorrect statement from the options below for the above cell:

