A heat pump is used to heat a building. The external temperature is less than the internal temperature. The pump's coefficient of performance is and the heat pump delivers as heat to the building each hour. If the heat pump is a Carnot engine working in reverse, at what rate must work be done to run it?

Important Questions on Entropy and the Second Law of Thermodynamics
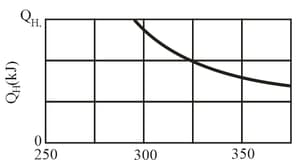
A Carnot engine is set up to produce a certain work per cycle. In each cycle, energy in the form of heat is transferred to the working substance of the engine from the higher-temperature thermal reservoir, which is at an adjustable temperature. The lower-temperature thermal reservoir is maintained at temperature Given figure gives for a range of The scale of the vertical axis is set by If is set at , what is
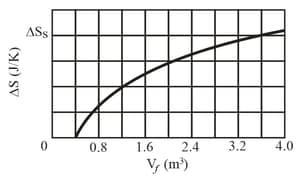
A gas sample undergoes a reversible isothermal expansion. Figure (as shown below) gives the change in the entropy of the gas versus the final volume of the gas. The scale of the vertical axis is set by . How many moles are in the sample?
Calculate the entropy change of the system during this process. (Heat of fusion for water is undefined) The system is then returned to the initial equilibrium state in an irreversible process (say, by using a Bunsen burner). Calculate the entropy change of the system during this process. Are your answers consistent with the second law of thermodynamics?
Suppose of a monatomic ideal gas is taken from initial pressure and volume , through two steps: an isothermal expansion to volume and a pressure increase to at constant volume. What is for step and step ? What is for step and step ? For the full process, what are and ?
The gas is returned to its initial state and again taken to the same final state but now through these two steps:
an isothermal compression to pressure and a volume increase to at constant pressure. What is for step and step ? What is for
step and step ? For the full process, what are and ?
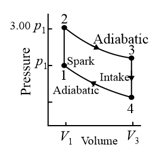
The cycle as shown in the figure represents the operation of a gasoline internal combustion engine. Volume . Assume the gasoline-air intake mixture is an ideal gas with What are the ratios (a), (b) , (c) , (d) and (e) ? (f) What is the engine efficiency?