A helium nucleus (charge mass ) is accelerated through a potential difference of . Calculate its kinetic energy in electronvolts.

Important Questions on Quantum Physics
A helium nucleus (charge mass ) is accelerated through a potential difference of . Calculate its kinetic energy in joules.
A helium nucleus (charge mass ) is accelerated through a potential difference of . Calculate its speed.
Ultraviolet light with photons of energy is shone onto a zinc plate. The work function of zinc is . Calculate the maximum energy with which an electron can be emitted from the zinc plate. Give your answer in eV.
Ultraviolet light with photons of energy is shone onto a zinc plate. The work function of zinc is . Calculate the maximum energy with which an electron can be emitted from the zinc plate. Give your answer in J.
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
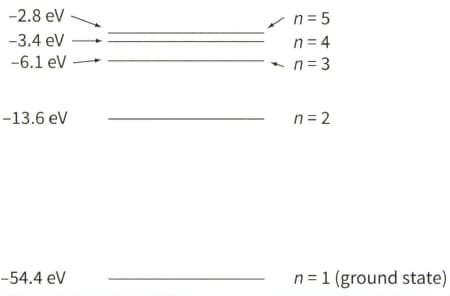
Determine the energy, in joules, that is required to completely remove the remaining electron, originally in its ground state, from the helium ion.
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
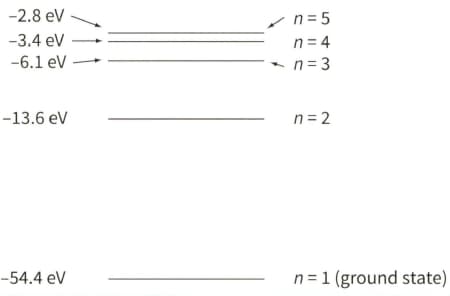
Determine the frequency of the radiation that is emitted when the electron drops from the level $n=3$ to $n=2$. State the region of the electromagnetic spectrum in which this radiation lies.
The diagram shows five of the energy levels in a helium ion. The lowest energy level is known as the ground state.
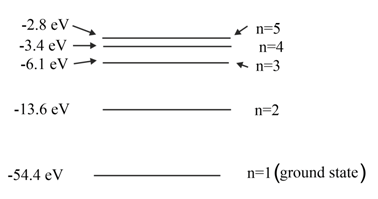
Without further calculation, describe qualitatively how the frequency of the radiation emitted when the electron drops from the level $n=2$ to $n=1$ compares with the energy of the radiation emitted when it drops from $n=3$ to $n=2$.
