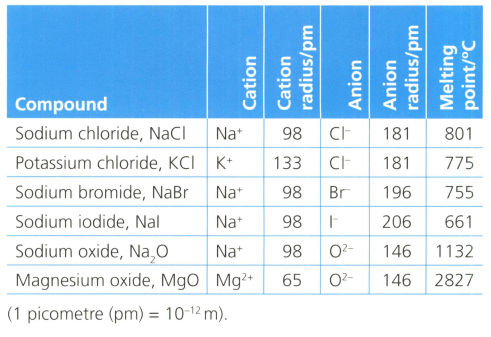
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
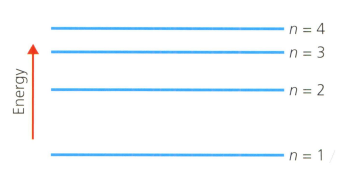
List these electron transitions in order of increasing wavelength of light absorbed or emitted:
- to
- to
- to
- to


Important Questions on Why do Electrons Matter?
A hydrogen atom has the main energy levels (shells) shown in the diagram below. refers to the shell number. This is the Bohr model of the atom.
Explain why a ladder is a good analogy or model for visualising the energy levels of atoms (and molecules), and what are the limitations of the analogy.
Analyse and evaluate the data about ionic compounds that are listed in the table below.
Identify and make scientifically supported judgments to explain any relationships between the size (radius) of ions present, and the predicted properties of their compounds. Your explanation should refer to your knowledge of ions, ionic bonding and the behaviour of electric charges. State any assumptions in your explanation.