MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A large increase in the rate of a reaction for a rise in temperature is due to
(a)increase in the number of collisions
(b)the increase in the number of activated molecules
(c)the shortening of mean free path
(d)the lowering of activation energy
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Which of the following statements are true regarding the plot shown in the given diagram?
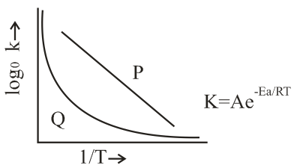
EASY
JEE Main/Advance
IMPORTANT
If the rate of reaction is given by: Rate
Which statements are correct?
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
