A light-sensitive compound 'X' of silver is used in black and white photography. On exposure to sunlight, its colour changes to grey. This is
reaction.
Important Questions on Chemical reactions and Equations
Write the balanced chemical equations for the chemical reaction; lead nitrate is heated. How can we confirm by the observation that these chemical reaction is taking place?
Complete the given chemical reaction:
.
Name the type of reaction.
Taking into consideration the relationship in the first pair, complete the second pair:
: Combination reaction: : _____.
The reaction of iron nail with copper sulphate solution is _____ reaction.
Answer the following:
In the chemical reaction given below:
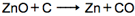
Name the substance which is reduced?
Which of the following reaction does not takes place? State the reason.
(i)
(ii)
The reaction of the iron nail with copper sulphate solution is a _____ reaction.
Answer the following:
In the chemical reaction given below:
Identify the oxidising agent.
(a) Identify the type of reaction and the gas X.
(b) Write balanced chemical equation of the reaction.
(c) Write the range of aqueous solution of the gas X.

