A metal cylinder of is heated to a steady temperature of and then dropped into a calorimeter of mass containing of water of . After stirring, the highest steady temperature of the mixture is observed to be . Write the value of specific heat capacity of the metal of the cylinder to the nearest integer(in ).
(specific heat capacity of water , specific heat capacity of calorimeter (copper) ).

Important Questions on Heat
A cube of ice of mass is dropped into of water at . Calculate the final temperature(in ) of the mixture. Round off answer to two decimal places.
Specific latent heat of fusion of ice, specific heat capacity of water.
Note: Write only numeric value.
Steam at is passed over a block of ice at . After sometime, of ice is left and of water at is formed. Calculate the specific latent heat of vaporisation of water in calorie per gram. (Specific latent heat of fusion of ice , specific heat capacity of water ).
A substance is in the form of a solid at . The amount of heat added to this substance and the temperature of the substance is plotted in the following graph:
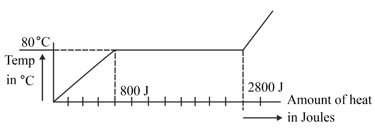
If the specific heat capacity of the solid substance is . Find the mass (in ) of the substance from the graph.
