MEDIUM
MHT-CET
IMPORTANT
Earn 100
A mixed ether on heating with dilute under pressure, gives mixture of two alcohols, viz., methanol and ethanol. In this reaction, yield of each alcohol is obtained. If the mass obtained for ethanol is , calculate the mass of the mixed ether used for the reaction.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Organic Reactions
MEDIUM
MHT-CET
IMPORTANT
The compound '', i.e., butyronitrile is subjected to the Stephen reaction by using and dilute , followed by the acid hydrolysis to give compound '', which is having a buttery odour, and it is used in margarine. The addition of hydrogen cyanide to the compound '', in the presence of a small amount of base, gives compound ''. Identify the compounds '' and ''.
EASY
MHT-CET
IMPORTANT
The compound i.e., propanamide is treated with bromine and alcoholic sodium hydroxide to give compound . The acid catalysed addition of valeraldehyde to compound yields compound which is well known as a 'Schiff base'. What are the structures of compounds and ?
MEDIUM
MHT-CET
IMPORTANT
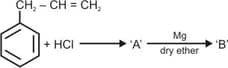
Identify the products and .
HARD
MHT-CET
IMPORTANT
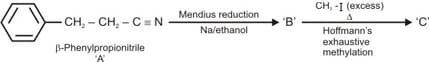
Predict the products in the following reaction:
MEDIUM
MHT-CET
IMPORTANT
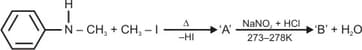
Predict the products in the following reactions:
EASY
MHT-CET
IMPORTANT
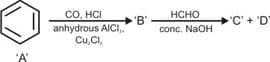
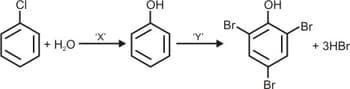
Identify '' and '' in the following example.
MEDIUM
MHT-CET
IMPORTANT
Bromobutane is treated with in the presence of dry acetone to give compound . The compound is boiled with moist silver oxide to give compound . Identify the compounds and . How many optical isomers are possible for compound ?
MEDIUM
MHT-CET
IMPORTANT
Identify and in the following reaction: