EASY
NEET
IMPORTANT
Earn 100
A mixture has of water and of ethanol. The mole fraction of water in the mixture is (assume ideal behaviour of the mixture)
(a)
(b)
(c)
(d)
42.86% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
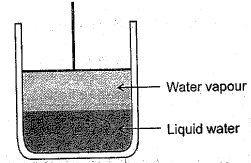
The vapour pressure of water at is What will be the vapour pressure of the water in the apparatus shown after the piston is lowered, decreasing the volume of the gas above the liquid to one half of its initial volume (assume temperature constant).