A mixture of gases (reactive) and (inert) is kept in a closed flask when A decomposes as per the following reaction at :
Vapour pressure of at is .
Calculate the half life for :
Calculate the half life for :

Important Questions on Chemical Kinetics
Which of the following is/are correct for the first order reaction?
(I) 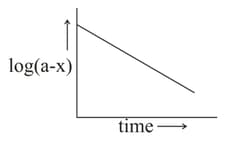
(II) 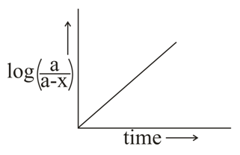
(III) 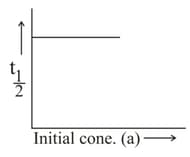
,
where and are the volume of standard needed to neutralize acid present at a given time. If ester is neutralised then
Consider the following reaction occurring in gas phase:
The reaction is experimentally found to obey the following rate law:
Step 1:
Step 2:
Step 3:
Based on this information, what conclusion can be drawn about this proposed mechanism:
Consider this gas phase reaction
The reaction is found experimentally to follow this rate law.
rate
Based on this information, what conclusions can be drawn about this proposed mechanism?
Step .
Step .
Step .
For a gas reaction at , the rate is given by . If the rate equation is expressed as ,, the rate constant is given by
(where, ideal gas law constant, )
