A mixture of and is heated with concentrated . The gas produced is collected and on treating with solution, the volume of the gas decreases by . Calculate the molar ratio of two acids in the original mixture. If the ratio comes out to be a:b, report your answer as 'a+b'.
Important Questions on Some Basic Concepts of Chemistry
In the following reaction:
of , atoms of and of reacted and formed of compound . If the atomic mass of and are and , respectively. What is the atomic mass of in ? (Avogadro number)
(Atomic weight: Mg = 24)
A gaseous hydrocarbon on combustion gives of water and . What is the empirical formula of the hydrocarbon?
(Given the molar mass of and molar mass of )
(Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5)
If of oxygen is burnt with of ethane. Calculate the volume of formed.
Galena (an ore) is partially oxidized by passing air through it at high temperature. After some time, the passage of air is stopped, but the heating is continued in a closed furnace such that the contents undergo self-reduction. The weight (in ) of produced per of consumed is ____. (Atomic weights in )
Give the answer to the nearest integer value.
(Assume complete combustion of reactant)
If the reaction sequence given below is carried out with moles of acetylene, the amount of the product formed (in ) is
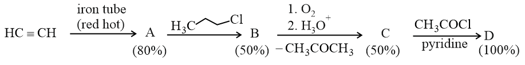
The yields of and are given in parentheses.
[Given: Atomic mass of ]
, identify di-hydrogen as a limiting reagent in the following reaction mixtures.
is used, even in spacecrafts, to produce The daily consumption of pure by a person in at atm, How much amount of in grams, is required to produce for the daily consumption of a person at atm, ?
Give answer to the nearest integer value.
The ammonia is prepared by treating ammonium sulphate with calcium hydroxide is completely used by to form a stable coordination compound. Assume that, both the reactions are completed. If of ammonium sulphate and of are used in the preparation, the combined weight (in grams) of gypsum and the nickel ammonia coordination compound produced is ____.
Atomic weights in :
In the following reaction
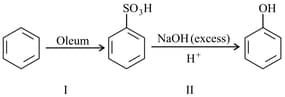
The yield for reaction is and that of reaction II is . The overall yield of the complete reaction is

