A mixture of hydrogen and oxygen has volume , temperature , pressure and mass . The ratio of number of moles of hydrogen to number of moles of oxygen in the mixture will be
[Take gas constant ]

Important Questions on Kinetic Theory of Gases
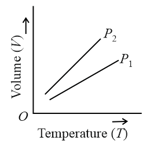
For a perfect gas, two pressures and are shown in figure. The graph shows
According to kinetic theory of gases,
A. The motion of the gas molecules freezes at .
B. The mean free path of gas molecules decreases if the density of molecules is increased.
C. The mean free path of gas molecules increases if temperature is increased keeping pressure constant.
D. Average kinetic energy per molecule per degree of freedom is (for monoatomic gases).
Choose the most appropriate answer from the options given below
Following statements are given
(1) The average kinetic energy of a gas molecule decreases when the temperature is reduced.
(2) The average kinetic energy of a gas molecule increases with increase in pressure at constant temperature.
(3) The average kinetic energy of a gas molecule decreases with increases in volume.
(4) Pressure of a gas increases with increase in temperature at constant pressure.
(5) The volume of gas decreases with increase in temperature.
Choose the correct answer from the options given below :
Same gas is filled in two vessels of the same volume at the same temperature. If the ratio of the number of molecules is , then
. The r.m.s. velocity of gas molecules in two vessels will be the same.
. The ratio of pressure in these vessels will be .
. The ratio of pressure will be .
. The r.m.s. velocity of gas molecules in two vessels will be in the ratio of .
