A mixture of pure and pure weighing was treated with excess of in acid medium. Iodine liberated required of hypo solution for exact oxidation. What is the percentage of each in the mixture? The reactions involved are:

Important Questions on Miscellaneous Problems for Revision
Under what pressure must an equimolar mixture of and be placed at in order to obtain conversion of into for is .
Round off your answer up to two places of decimal.
of an organic compound , on ignition, gives and on boiling with and adding solution gives of . The vapour density of is on hydrolysis with yields , which on mild reduction gives an optically active compound . On heating with and , iodoform is produced along with . With , gives a solid, which is markedly more soluble in hot water than in cold. Identify and .
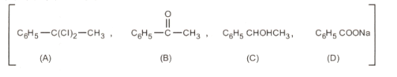
Two organic compounds containing , with and , gave compounds, which on hydrolysis gave two isomeric monobasic acids with molecular mass Name the compounds and also the third isomer.
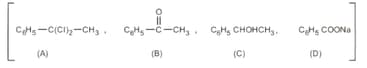
The compound , with molecular formula , does not react appreciably with Lucas reagent at room temperature, but gives a precipitate with ammonium silver nitrate. With an excess of of gives of at . The treatment of with in the presence of catalyst, followed by boiling with an excess , gives pentane. Suggest the structure for .
