EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
A molecule whose molar specific heat at high temperature, assuming ideal behaviour, is is:
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Thermodynamics & Thermochemistry
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The maximum amount of work produced by a heat engine operating between and , if of heat is absorbed from the hot reservoir, is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The ratio of the heat capacities for one mole of a gas is . The gas is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
A cylinder of cooking gas in a household contains of butane. The thermochemical reaction for the combustion of butane is . If the household needs of energy per day, the cooking gas cylinder will last for about:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Human being required of energy per day
Answer the following:
(i) Human being required ...... of energy per day.
(ii) Amount of sucrose required per day and volume of evolved during the process [at ].
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A concentrated solution of copper sulphate, which is dark blue in colour, is mixed at room temperature with a dilute solution of copper sulphate, which is light blue. For this process:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
For the reaction and , the value of and the temperature, at which the reaction reaches equilibrium are, respectively.
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Diborane is formed from the elements as shown in equation .
Given that, ...
the for the reaction is:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An isolated box, equally partitioned contains two ideal gases and as shown:
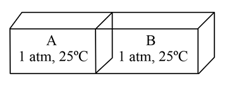
When the partition is removed, the gases mix. The changes in enthalpy and entropy in the process, respectively, are:
