A natural linear polymer of -methyl-, -butadiene becomes hard on treatment with sulphur between to and bonds are formed between chains. Write the structure of the product of this treatment?

Important Points to Remember in Chapter -1 - Polymers from NCERT NCERT Exemplar Chemistry - Class 12 Solutions
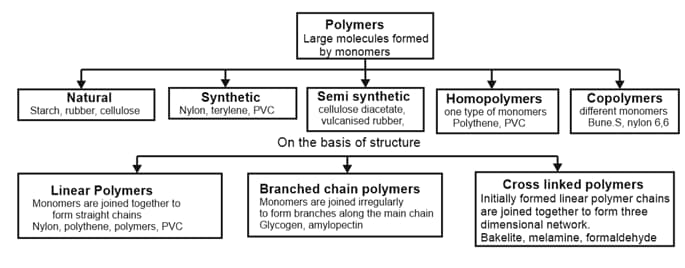
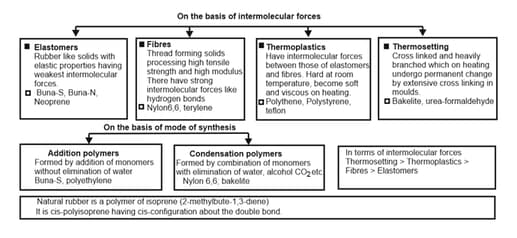
2. Types of Polymerisation Reactions:
There are two broad types of polymerisation reactions, i.e., the addition or chain growth polymerisation and condensation or step growth polymerisation.
3. Natural rubber:
Rubber is a natural polymer and possesses elastic properties. It is also termed as elastomeric polymer. In elastomeric polymers, the polymer chains are held together by the weak intermolecular forces.
4. Synthetic rubbers:
Neoprene, Buna-S and Buna-N are examples of synthetic rubbers.
5. Biodegradable Polymers:
(i) Poly -hydroxybutyrate – co--hydroxy valerate (PHBV): It is obtained by the copolymerisation of -hydroxybutanoic acid and - hydroxypentanoic acid.
(ii) Nylon –nylon-: It is an alternating polyamide copolymer of glycine and amino caproic acid and is biodegradable.
