MEDIUM
NEET
IMPORTANT
Earn 100
A non-volatile, non-electrolyte solute is dissolved in a suitable solvent and the osmotic pressure ( in ) of solutions of various concentration in is measured at The slope of the plot against is found to be The molecular weight of is:
(a)
(b)
(c)
(d)
26.04% studentsanswered this correctly

Important Questions on Solutions
EASY
NEET
IMPORTANT
The net change of for ferrous sulphate solution on addition of ,
is from:
(Assume complete consumption of all reactants)
MEDIUM
NEET
IMPORTANT
Among the following, the solution which shows the highest osmotic pressure is: (assume all of them to be 100% dissociated)
MEDIUM
NEET
IMPORTANT
What happens when mercuric iodide is added to an aqueous solution of ?
HARD
NEET
IMPORTANT
What are the minimum values of the Van't Hoff factors in a solute undergoing dimerisation and trimerisation?
HARD
NEET
IMPORTANT
Which of the following pair of solutions are expected to be isotonic at the same temperature?
HARD
NEET
IMPORTANT
Study the following figure and choose the correct options.
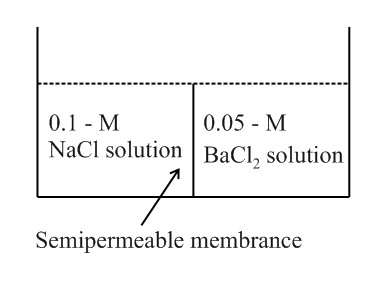
HARD
NEET
IMPORTANT
Which of the following pair of solutions have the same values of the Van't Hoff factor? (assume all of them are undergoing complete dissociation)
MEDIUM
NEET
IMPORTANT
Which of the following statement is incorrect regarding Henry's law?
