A nucleus of an element emits an -particle first, -particle next and then a gamma photon. The final nucleus formed has an atomic number.
Important Questions on Nuclear Physics
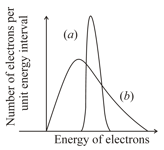
Originally the radioactive beta decay was thought as a decay of a nucleus with the emission of electrons only (Case I) . However, in addition to the electron, another (nearly) massless and electrically neutral particle is also emitted (Case II). Based on the figure below, which of the following is correct?
Assertion (A) : Neutrino is chargeless and possesses spin.
Reason Neutrino exists inside the nucleus.
Carbon decays to boron according to the following formula: .
Assume that positrons produced in the decay combines with free electrons in the atmosphere and annihilate each other almost immediately. Also, assume that the neutrinos are massless and do not interact with the environment. At , we have of . If the half-life of the decay process is , the net energy produced between time, and will be nearly,
In radioactive reaction,
Radioactive radiation are emitted in the sequence:

