A nucleus of beryllium- decays into an isotope of boron by $\beta^{-}$ emission. The chemical symbol for boron is $\mathrm{B}$.
(a) Write a nuclear decay equation for the nucleus of beryllium-10.

Important Questions on Nuclear Physics
A nucleus of beryllium- decays into an isotope of boron by $\beta^{-}$ emission. The chemical symbol for boron is $\mathrm{B}$.
(b) Calculate the energy released in this decay and state its form.
(a) Explain why hydrogen (proton) cannot appear on the graph shown in Figure.
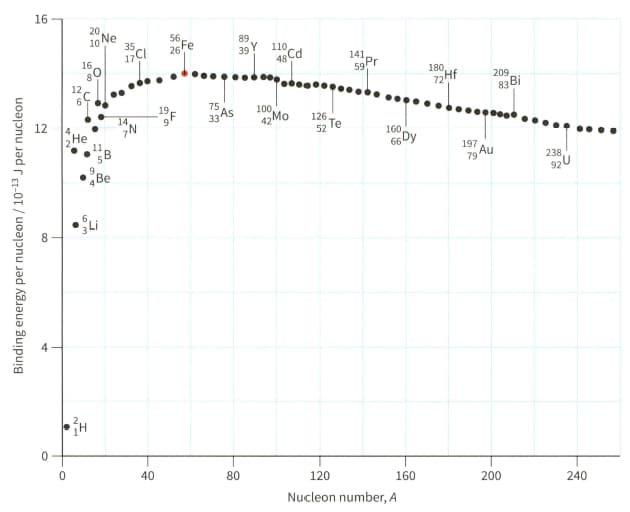
(b) Use Figure to estimate the binding energy of the nuclide .
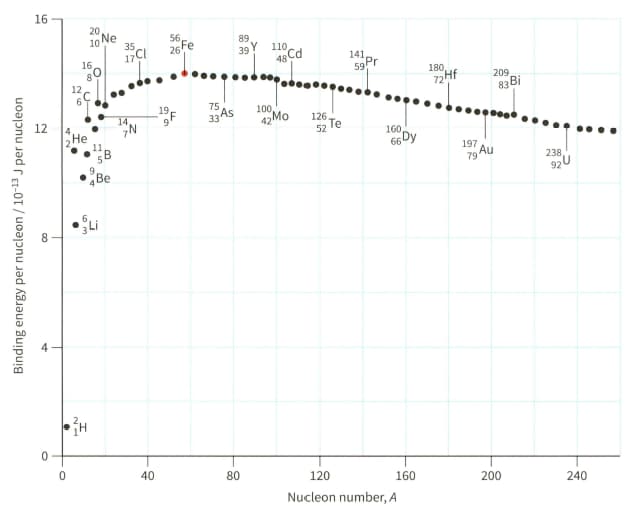
The mass of Be nucleus is . For the nucleus of , determine:
(a) the mass defect in $\mathrm{kg}$
The mass of Be nucleus is . For the nucleus of , determine:
(b) the binding energy of the nucleus in $\mathrm{MeV}$
The mass of Be nucleus is . For the nucleus of , determine:
(c) the binding energy (in $\mathrm{MeV}$ ) per nucleon for the nucleus.
Use the binding energy graph (Figure) to suggest why fission is unlikely to occur with 'light nuclei' $(A<20)$ and why fusion is unlikely to occur for heavier nuclei $(A>40)$.
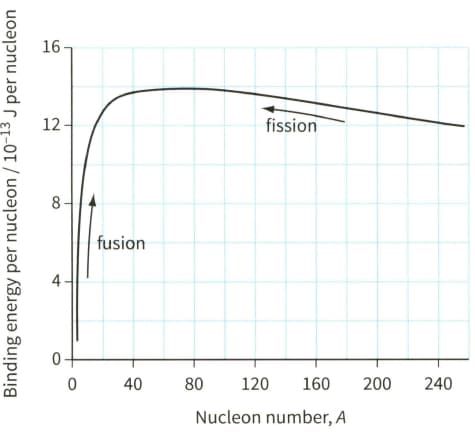
Use the information given in the fusion section, to determine the binding energy (in ) per nucleon of each particle in the following fusion reaction: .
(Use:The binding energy of deuterium nucleus is , and the binding energy of helium- nucleus is .)
